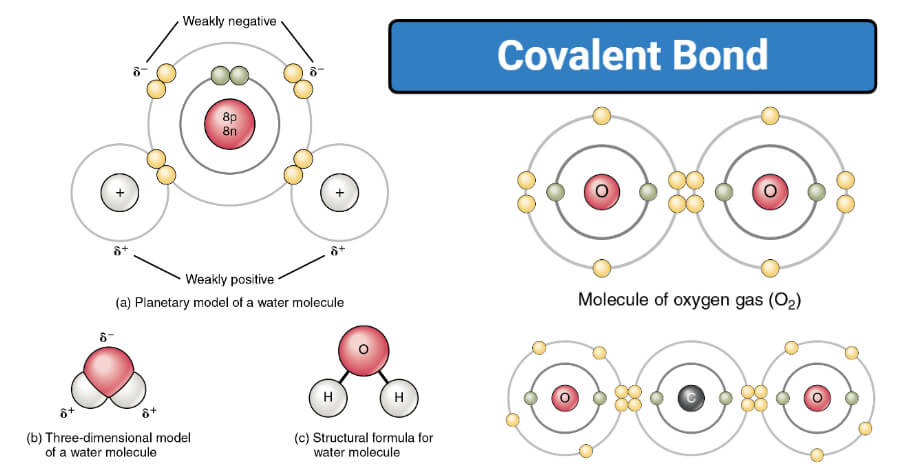
Describe the General Location of Electrons in a Covalent Bond
The first shell n 1 can hold 2 electrons in one orbital labelled 1s. An atom that shares one or more of its.
Covalent Bonds Study Guide Inspirit
Adams Adams share electrons in order to minimize the potential energy and become more stable.

. Atoms can also make chemical bonds by sharing electrons equally between each other. C - O the bond is called a polar covalent bond and the. Polar-covalent bond - unequal attraction for the electron pair resulting in one of the bonded atoms possessing a partial negative charge and the other atom possessing a partial positive charge.
Describe the general location of electrons in a covalent bond. C-C the bond is called a nonpolar covalent bond. Describe the general location of the electrons in a covalent bond.
If the atoms that form a covalent bond are identical as in H 2 Cl 2 and other diatomic molecules then the electrons in the bond must be shared equally. Covalent bond when electrons are shared. Non-polar covalent is when the bonding electrons are shared equally by the bonded atoms resulting in a balanced distribution of electrical charge.
The electrons as fuzzy clouds around the nuclei of the molecule have kinetic energy not only electrostatic. They lie between the two nuclei of the bonding atoms. A brief treatment of covalent bonds follows.
In ionic compounds electrons are transferred between atoms of different elements to form ions. The binding arises from the electrostatic attraction of their nuclei for the same electrons. The location of N in the periodic table is shown below.
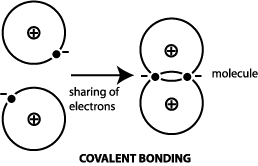
The two atoms in the bond equally share the electrons. Such bonds are called covalent bonds. -Covalent bonds in which bonded electrons are shared unequally between participating atoms are polar covalent bonds Example.
A covalent bond whose electron density is concentrated in the region directly between the nuclei. If the atoms that form a covalent bond are identical as in H 2 Cl 2 and other diatomic molecules then the electrons in the bond must be shared equallyWe refer to this as a pure covalent bondElectrons shared in pure covalent bonds have an equal probability of being near each nucleus. The great contributions of Hellmann and then Ruedenberg and co-workers 49505152535455565758597383 were to focus attention on the role of quantum mechanical kinetic energy in the energetic analysis of covalent bonding.
Chlorine more negative and hydrogen more positive. In ionic compounds electrons are transferred between atoms of different elements to form ions. Describe the general location of the electrons in a covalent bond.
The shared electrons are typically near the middle of the bond between the 2 atoms in a covalent bond. In a covalent bond electrons are shared between the two atoms that come together to form said bond. A covalent bond may also be termed a molecular bond.
A region in space around the atoms nucleus where there is a probability of finding an electron. We refer to this as a pure covalent bond. This type of bond may also be found in other chemical species such.
But this is not the only way that compounds can be formed. When the pair of electrons are shared by atoms that have the same electronegativity ie. Each electron is free to occupy either of the.
They occupy overlapping orbitals. The general location of electrons in a covalent bond is that electrons are shared in pairs between 2 atoms. 1112017 100805 PM To Bond or Not to Bondp.
They may be slightly closer to 1 atom or the other due to small differences in electronegativity. In general the shared electrons between two atoms are shared near the middle of the bond. If 2 electrons pairs are shared 4 electrons are shared in all.
Vaillant Bond is a bond that is formed by the sharing of a pair of electrons by two. Such bonds are called covalent bonds. They are in overlapping orbitals in the middle of two atoms.
Two atoms with either equal or very similar electronegativity values the difference must not exceed 05 come together to form a nonpolar covalent bond. Ionic bonding results from the electrostatic attraction of oppositely charged ions that are typically produced by the transfer of electrons between metallic and nonmetallic atoms. Describe the Created Date.
The general location of electrons in a covalent bond is that electrons are shared in pairs between 2 atoms. 81 360 ratings A 0. But this is not the only way that compounds can be formed.
Covalent bond in chemistry the interatomic linkage that results from the sharing of an electron pair between two atoms. B In metals the valence electrons are considered to be a attached to particular positive ions. A covalent bond forms when two non-metal atoms share a pair of electrons.
A covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. A covalent bond in chemistry is a chemical link between two atoms or ions in which the electron pairs are shared between them.
Covalent bonds are formed between two atoms when both have similar. Determine the number of electrons donated by other atoms. A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons.
Atoms can also make chemical bonds by sharing electrons equally between each other. The electrons involved are in the outer shells of the atoms. However the nature of those atoms will determine if those electrons are shared equally or unequally.
1 day agoThe chemistry of nitrogen is dominated by the ease with which nitrogen atoms form double and triple bonds. Identify Sigma Bonding Answer the following questions about SO3. If 2 electrons pairs are shared 4 electrons are shared in all.
Covalent bonds are formed between two atoms when both have similar. Describe the general location of the electrons in a covalent bond. On the other hand when the bond contains two atoms of different electronegativity ie.
1 day agoThe bonding region is the location EXAMPLE PROBLEM. Covalent bonds form between two nonmetal atoms with identical or relatively close electronegativity values. The atoms might be slightly closer to one Adam thin the other due to.
A different type of bonding results from the mutual attraction of atoms for a shared pair of electrons. Such bonds are called covalent bonds.

Covalent Bonding 1 3 7 Cie A Level Chemistry Revision Notes 2022 Save My Exams

Covalent Bond Definition Properties Examples Facts Britannica
Ces Information Guide Materials Science Engineering

Covalent Bonding 1 3 7 Cie A Level Chemistry Revision Notes 2022 Save My Exams
No comments for "Describe the General Location of Electrons in a Covalent Bond"
Post a Comment